Clinical Trials
Information on how to find clinical trials and available resources.
- Application of Good Clinical Practice
This guidance describes Good Clinical Practice (GCP), provides investigators, sponsor-investigators, and study teams with a decision tree to determine if their research must follow the standards described in GCP, and includes detailed guidance about what it means to follow GCP.
What is Good Clinical Practice (GCP)? The term GCP is used interchangeably in reference to both a broad concept and a single document.
GCP is often used broadly to describe what are considered best practices when conducting clinical research. These practices set the standards for designing, conducting, performance monitoring, auditing, recording, analysis, and reporting of clinical research that involve human subjects. Compliance with this standard helps to provide public assurance that the rights, safety, and welfare of human subjects in research are protected; that the research is consistent with the principles that have their origin in the Declaration of Helsinki; and that the data collected are reliable and credible.
The term GCP is also used more narrowly to describe the International Council for Harmonisation (ICH) GCP E6 document itself. GCP describes the unified, international standard for the European Union, Japan, the United States, Canada, and Switzerland to facilitate the mutual acceptance of data from clinical trials by the regulatory authorities in these jurisdictions.
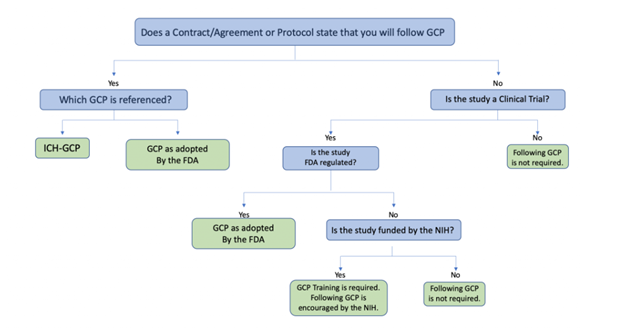
The applicability and application of GCP differs based on the specifics of the study. Use the decision tree below to help determine if GCP applies to your study.
Decision tree data:
Does a contract/agreement or protocol state that you will follow GCP?
- Yes, it states that GCP will be followed
- Which GCP is referenced?
- ICH-GCP
- GCP as adopted by the FDA
- Which GCP is referenced?
- No, it does not state the GCP will be followed
- Is the study a clinical trial?
- Yes, the study is a clinical trial
- Is the study FDA regulated?
- Yes, the study is FDA regulated
- GCP as adopted by the FDA
- No, the study is not FDA regulated
- Is the study funded by the NIH?
- Yes, the study is funded by the NIH
- GCP training is required. Following GCP is encouraged by the NIH
- No, the study isn't funded by the NIH
- Following GCP is not required
- Yes, the study is funded by the NIH
- Is the study funded by the NIH?
- Yes, the study is FDA regulated
- Is the study FDA regulated?
- No, the study isn't a clinical trial
- Following GCP is not required
- Yes, the study is a clinical trial
- Is the study a clinical trial?
What is a clinical trial?
A clinical trial is defined by NIH as: A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.How are ICH GCP and GCP as adopted by the FDA related?
ICH GCP Guidelines should be followed when generating clinical trial data that are intended to be submitted to international regulatory authorities. In protocols or contracts/agreements, when the international standards are to be followed, it is referred to as ICH GCP.
The United States Food and Drug Administration (FDA) adopted the ICH GCP E6 (R2) Integrated Addendum as guidance in March 2018. FDA regulated clinical trials must follow the aspects of GCP that are consistent with the applicable FDA regulations. The aspects of GCP that are not otherwise described in the FDA regulations are considered guidance, similar to other FDA guidance. Refer to the table at the end of this document.
In protocols or contracts/agreements, GCP as adopted by the FDA may also be referred to as: Good Clinical Practice, GCP, ICH GCP as adopted by the FDA, GCP as ratified by the FDA, or GCP as described in the FDA Regulations.
FDA regulated clinical trials are those using an investigational product. The term investigational product includes drugs, biologics, devices and diagnostics when the objectives of the research involve assessing the safety or efficacy of the agent or pursuing approval from the FDA for a new indication. In some cases, dietary supplements and food additives can be considered investigational products. Any study involving an investigational drug, biologic or device is considered FDA-regulated, including IND Exempt and Non-Significant Risk determinations.
As it pertains to the conduct of clinical trials using an investigational product involving human subjects, study teams are expected to apply GCP encompassed within the applicable FDA regulations including 21 CFR 11, 50, 54, 56, 312, 812 and 814 (subpart H).
Does the National Institutes of Health (NIH) require that I follow GCP on studies for which they provide funding?
NIH encourages researchers to follow GCP as best practice for NIH funded clinical trials but does not currently require that clinical trials comply with GCP. The NIH requires training in GCP to help ensure the safety, integrity, and quality of clinical trials.
What does it mean to follow GCP as adopted by the FDA?
To follow GCP as adopted by the FDA means to follow GCP guidelines that have a corresponding, applicable FDA regulation. As noted previously in this document, application of GCP guidelines that do not have an exact corresponding applicable FDA regulation is optional (refer to statement above from FDA regarding the purpose of FDA guidance documents). The table below includes those requirements that are unique to ICH GCP, but do not have a corresponding FDA regulation. All other GCP guidelines have an applicable FDA regulation and should be applied.
What if I am supposed to follow GCP as adopted by the FDA, but don’t?
GCP “as adopted by the FDA” means GCP guidelines that have a corresponding, applicable FDA regulation. You are required to follow GCP as adopted by the FDA if you are conducting FDA regulated research or if the clinical trial agreement requires you to follow GCP as adopted by the FDA. Failure to follow GCP as adopted by FDA in these cases could result in findings of noncompliance, a report to the FDA, or a breach of contract, which could put the university at risk of monetary penalties and which may be assessed to your department, among other results.
If I am conducting an FDA regulated trial, do I need to follow GCP criteria that are not within FDA Regulations?
The FDA recommends following GCP guidelines within its guidance documents. In the Introduction of the FDA’s guidance on GCP, it states, “In general, FDA’s guidance documents do not establish legally enforceable responsibilities. Instead, guidances describe the Agency’s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required.”
OSU-CHS practices for citing findings are aligned with FDA expectations as noted above. As such, if a study is not FDA regulated and there is no contract requiring application of ICH GCP criteria that have not been adopted by FDA, study teams will not be cited for not following ICH GCP guidelines that are unique to ICH GCP (i.e., those that do not appear in FDA regulations) unless the lack of doing so presents a more significant potential impact on subject safety or data integrity.
What if my contract or protocol states that ICH GCP will be followed? How does ICH GCP compare to FDA regulations?
The ICH GCP guidelines generally agree with the FDA regulations, but there are a few areas in which ICH GCP guidelines have requirements that go beyond the FDA regulations and are considered unique to ICH GCP.
By stating or agreeing to follow ICH GCP guidelines, you are committing to following guidelines in addition to those consistent with the FDA regulations. If your study is supposed to follow ICH GCP guidelines, but does not, you could be cited accordingly.FDA and/or ICH GCP Section/Reference Description ICH GCP E6 4.8.8 and 21 CFR 50.27(a) ICH GCP Guidance states that “prior to a subject’s participation in the trial, the written informed consent form should be signed and personally dated by the subject or by the subject’s legally acceptable representative, and by the person who conducted the informed consent discussion.”
The FDA regulations (50.27[a]) (Protection of Human Subjects 2016) only require the signature of the subject and the date the subject signed the consent form.ICH GCP E6 4.8.11 and FDA reference ICH GCP Guidance requires that the subject or the legally acceptable representative (LAR) receive a signed and dated copy of the written informed consent form.
The FDA regulations allow subjects to receive either a signed or unsigned copy, as long as it is the IRB-approved version of the consent form.ICH GCP E6 4.3 ICH GCP Guidelines require a qualified physician (or dentist when appropriate) investigator (or sub-investigator) to be responsible for trial-related medical (or dental) decisions.
The FDA does not explicitly require this.ICH GCP D6 4.9.0, multiple 21 CFR 312 and 812 references The FDA regulations require records be adequate and accurate, however ICH GCP guidelines are more detailed, and require records be attributable, legible, contemporaneous, original, accurate, and complete (ALCOAC). However, the FDA Compliance Program Bioresearch Monitoring Manual 7348.811 for the inspection of Clinical Investigators and Sponsor-Investigators describes the document requirements as attributable, legible, contemporaneous, original, and accurate (ALCOA). ICH GCP E6 5.5 and multiple 21 CFR 312 and 812 references FDA regulations describe that the sponsor is responsible for trial management, data handling, and record keeping. However, ICH 5.5.3 also requires the sponsor to ensure that the electronic trial data handling
and/or remote electronic trial data systems conform to the sponsor’s established requirements for completeness, accuracy, reliability, and are validated and that sponsors maintain SOPs for these systems.ICH GCP E6 8.1 ICH GCP Guidelines describe the documents that are considered Essential Documents and conditions under which they should be stored and retained. Specifically, ICH GCP describes:
- Sponsor and investigator should maintain a record of the location(s) of their respective essential documents, including source documents. The storage system should provide for document identification, search and retrieval.
- When a copy is used to replace an original document, it should fulfill the requirements for certified copies.
ICH GCP E6 Section 8 The use of a Delegation of Authority Log is described in GCP, but not explicitly in the FDA Regulations. It is, however, described in the FDA Compliance Program Bioresearch Monitoring Manual 7348.811 followed by the FDA inspectors. Applicable to Sponsor-Investigators ICH GCP 5.2.2 and 21 CFR 312.52 Both the ICH GCP Guidelines and federal regulations allow the sponsor to delegate (in writing) responsibilities to a contract research organization (CRO), but ICH GCP also requires that the sponsor ensure oversight even if duties are subcontracted to a CRO. FDA regulations state that if all obligations are transferred, a general statement that all obligations have been transferred is acceptable. ICH makes no such mention of a general statement being acceptable. ICH GCP E6 5.0 ICH GCP Guidelines state that the sponsor should implement a system to
manage quality throughout all stages of the trial process, and the quality
management system should use a risk-based approach, including
identification of study risks to determine which may safely be omitted from
continual monitoring.ICH GCP E6 5.18.3 ICH GCP Guidance states that the sponsor should:
- Develop a systematic, prioritized risk-based approach to monitoring.
- The sponsor may choose on-site monitoring, a combination of on-site and centralized monitoring, or where justified, only centralized monitoring.
- Centralized monitoring, also referred to as remote monitoring, is a remote evaluation of accumulating data, performed in a timely manner, supported by appropriately qualified and trained persons.
- Document the rationale for the chosen monitoring strategy.
ICH GCP E6 5.18.7 and 21 CFR 312.50 ICH GCP Guidance requires sponsors to develop a monitoring plan tailored to the specific human subject protection and data integrity risks of the trial.
The FDA Regulations only state that the sponsor is responsible for ensuring proper monitoring of the investigation.ICH GCP E6 5.20.1 ICH GCP Guidance requires the sponsor to take prompt action to secure compliance when noncompliance with the protocol, SOPs, GCP, and/or
applicable regulatory requirements is discovered. ICH E6 further requires the sponsor to perform a root cause analysis and implement an appropriate corrective and preventive action depending on the significance of the noncompliance. The FDA Regulations do not explicitly describes these tasks.ICH GCP E6 5.20.2 ICH GCP Guidance states that if the sponsor identifies continuing and/or serious noncompliance, the sponsor should end the investigator’s/institution’s participation in the clinical trial and notify regulatory Authorities.
Also described in 7348.811 Bioresearch Monitoring Manual followed by the FDA inspectors, Section M. Monitoring
2c. Determine if the study records include a log of on-site monitoring visits, written reports or other communication provided to the clinical investigator. Obtain a copy of the log (if any) and examples of monitor reports and communications; and
2d. Follow up activities performed by the clinical investigator when the monitor(s) found deficiencies or recommended changes, for example, in the conduct of the study or records associated with the study.
3. For sponsor-investigators, determine if any monitoring was done for the study and, if so, describe. Obtain a copy of the monitoring SOP, if available. - Yes, it states that GCP will be followed
- Good Clinical Practice Training
Good Clinical Practice (GCP) is an international ethical and scientific quality standard for designing, conducting, recording, and reporting clinical trials. It ensures the rights, safety, and well-being of participants and the credibility of clinical trial data.
GCP outlines the responsibilities of investigators, sponsors, monitors, and Institutional Review Boards (IRBs) and applies to both biomedical and behavioral research.Why GCP Matters
GCP ensures:
- Compliance with ethical and regulatory standards.
- Accurate and reliable data collection, monitoring, and reporting.
- Protection of human subjects throughout the research process.
Who Must Complete GCP Training?
Good Clinical Practice (GCP) training is mandatory for all personnel involved in NIH-funded clinical trials, industry-sponsored clinical trials that explicitly require GCP training in the clinical trial agreement, or clinical trials subject to FDA regulations . This includes individuals who:
- Design, conduct, oversee, or manage clinical trials.
- Recruit or consent participants.
- Collect or manage study data.
- Perform study-related procedures or assessments.
- Access identifiable participant data or biospecimens
- Contribute significantly to data management
Examples of required personnel:
- Principal Investigators
- Study Coordinators/Research Nurses/Clinical Research Coordinators
- Sub-Investigators
- Data Managers and Statisticians
Note: These personnel should be listed in the IRB application.
Local healthcare providers (HCPs) may support certain trial-related activities—such as routine clinical assessments or procedures - when these align with their standard scope of practice and do not require detailed knowledge of the study protocol, investigational product, or investigator’s brochure. According to FDA guidance on decentralized clinical trials, these providers are not considered study personnel or sub-investigators if their involvement is limited to standard clinical care or delegated tasks that do not involve investigational oversight or data interpretation. Such providers are not required to complete GCP training, provided their role does not extend beyond routine care or delegated procedures that fall within their existing qualifications and licensure. However, sponsors and investigators must ensure that any trial-related activities performed by local HCPs are appropriately documented, and that these individuals are trained for their specific responsibilities if those responsibilities go beyond standard care.
FAQs
What is a clinical trial?
A clinical trial is defined by NIH as: A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.How often do I have to complete GCP training?
GCP training completed in CITI must be completed every three years. The clinical trial sponsor may require training more frequently, as specified in the clinical trial agreement.How do I refresh my GCP training?
GCP training must be renewed every three years through completing the CITI GCP “refresher module” that is available for those who have completed the initial CITI GCP training. For those taking the GCP training module, the refresher course will auto-populate in the individual’s course menu 90 days before the training expiration date. CITI will send reminder emails beginning 90 days before the GCP expiration in CITI.Does the ICH E6 Good Clinical Practice (GCP) guideline mandate GCP training for clinical trial personnel?
The ICH E6 Good Clinical Practice (GCP) guideline does not explicitly mandate GCP training, but it strongly implies the need for it by emphasizing the qualifications and responsibilities of personnel involved in clinical trials.
Here’s what the ICH GCP guideline says:- Section 2.8: “Each individual involved in conducting a trial should be qualified by education, training, and experience to perform his or her respective task(s).”
- Section 4.1.1: “The investigator should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial...”
- Section 5.6.1: Sponsors are responsible for ensuring that trial-related duties and functions are delegated to appropriately trained individuals
So, while ICH GCP does not prescribe a specific training course, it clearly requires that all personnel involved in a clinical trial be appropriately trained to ensure compliance with GCP standards. In practice, this is often fulfilled through formal GCP training programs, such as those offered by CITI or provided by a sponsor.
- CenterWatch - Clinical Trials Listing Service™
Use CenterWatch to find a wealth of information about clinical research, including listings of more than 41,000 active industry and government-sponsored clinical trials, as well as new drug therapies in research and those recently approved by the FDA. This site is designed to be an open resource for patients interested in participating in clinical trials and for research professionals.
- ResearchMatch
ResearchMatch is a nonprofit program funded by the National Institutes of Health (NIH). It helps to connect people interested in research studies with researchers from top medical centers across the U.S.
ResearchMatch is only available to OSU-CHS faculty, staff, and students working under projects approved by the OSU-CHS Institutional Review Board. - Resources
- Stipend Cards (ClinCards)
All requests for study subject stipend cards must be submitted through our online forms. OSU-CHS Clinical Trial utilizes Greenphire ClinCards to provide participants with compensation for their participation in approved studies.
New Study or Amendment Build Request
Please use this form to request the set-up of a new study in the Greenphire ClinCard System or to make edits to an existing study. This request form does not include any physical card disbursement request. If you do not already have access to the Greenphire system, please see the next form. Access to the system is a required feature for studies using ClinCards.
Greenphire New User Access Form
Please use this form to request a new user account or to edit an existing account. Access to the ClinCard system can be granted to the PI, research team, or any staff member and is required for the distribution of ClinCards.
Physical Stipend Cards Request Form
Please use this form to request physical cards to distribute to study participants. The initial study stipend card request form does not contribute to receiving physical cards.
- ClinicalTrials.gov
Background
ClinicalTrials.gov is a Web-based resource that provides patients, their family members, health care professionals, researchers, and the public with easy access to information on publicly and privately supported clinical studies on a wide range of diseases and conditions. Most of the records on ClinicalTrials.gov describe clinical trials (also called interventional studies). A clinical trial is a research study in which human volunteers are assigned to interventions (for example, a medical product, behavior, or procedure) based on a protocol (or plan) and are then evaluated for effects on biomedical or health outcomes. ClinicalTrials.gov also contains records describing observational studies and programs providing access to investigational drugs outside of clinical trials (expanded access). Studies listed in the database are conducted in all 50 States and in 216 countries. ClinicalTrials.gov does not contain information about all the clinical studies conducted in the United States because not all studies are required by law to be registered (for example, observational studies and trials that do not study a drug, biologic, or device).
How to search studies:
- To explore the registered studies from all 50 states and 216 countries, go here to search by condition or disease.
- To see current studies being conducted at Oklahoma State University, go here.
Relevancy
Certain FDA-regulated studies, NIH-funded studies and other unfunded studies with an intent to publish in an ICMJE journal require registration in ClinicalTrials.gov.
FDA Registration Requirements
The Food and Drug Administration (FDA) requires registration at ClinicalTrials.gov for all applicable clinical trials (ACTs) that were initiated after 9/27/2007, or were initiated before 9/27/2007, but were ongoing as of 12/26/2007. Applicable drug and device clinical trials are defined as follows:
Drugs and Biologics: A controlled clinical investigation, other than a Phase 1 clinical investigation, of a drug or biologic product subject to FDA regulation.
Medical Devices: A prospective clinical study of health outcomes comparing an intervention with a medical device against a control, or pediatric post market surveillance required by the FDA.
Registration is not required for small trials to determine the feasibility of a device or to test prototype devices where the primary outcome measure relates to feasibility, and not to health outcomes.
A checklist for determining whether your FDA regulated study is required to b e registered at ClinicalTrials.gov can be found here
NIH Registration Requirements
Effective January 18, 2017, National Institutes of Health requires registration of funded Clinical Trials (fully or partially funded). NIH defines a clinical trial as: a study where one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
The following questions should be used to determine whether a study meets the NIH clinical trial definition:
- Does the study involve human participants?
- Are the participants prospectively assigned to an intervention?
- Is the study designed to evaluate the effect of the intervention on the participants?
- Is the effect being evaluated a health-related biomedical or behavioral outcome?
If the answers are all “yes,” the study is a clinical trial.
NOTE: If the answer to all four questions are yes, your study meets the NIH definition, even if:
- You are studying healthy participants;
- Your study is a single intervention with no comparison group (e.g. placebo or control)
- Your study is only designed to assess the pharmacokinetics, safety, and/or maximum tolerated dose of an investigational drug; and
- Your study is utilizing a behavioral intervention.
Checklists for determining whether your NIH-funded study is required to be registered at ClinicalTrials.gov can be found here and here.
ICJME Registration Requirements
In 2005, member publications of the International Committee of Medical Journal Editors (ICMJE) required that all clinical trials be registered. The ICMJE defines a clinical trials as:
“…any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes. Health-related interventions include any intervention used to modify a biomedical or health-related outcome (for example, drugs, surgical procedures, devices, behavioral treatments, dietary interventions, and process-of-care changes). Health outcomes include any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events."
For trials that began enrolling participants on or after January 1, 2019, ICMJE requires a data sharing statement; information about that statement can be found here.
Information to help determine whether your study is required to be registered at ClinicalTrials.gov is available here.
How are clinical trials registered?
Clinical trials are registered via a web-based protocol registration system (PRS). Instructions for completing registration can be found here.
Setting up an account can take time, so plan accordingly.
Important: The Oklahoma State University CHS PRS Account Administrator role is to help establish an account. The PRS Account Administrator is not responsible for registering, ensuring compliance with timelines, entering results or verifying accuracy of information.
Who is responsible for registering a study?
It is important for research to know who the “responsible party” is. Penalties can be levied against the responsible party for failing to meet requirements. Penalties can include:
- Monetary fines;
- Withholding federal funds;
- Denial of publication in an ICMJE journal.
Responsible party definitions -
FDA-regulated studies
- The sponsor of the clinical trial, or
- The principal investigator (PI) of such clinical trial if so designated by the sponsor, grantee, contractor, or awardee, so long as the PI is responsible for conducting the trial, has access to and control over the data from the clinical trial, has the right to publish the results of the trial, and has the ability to meet all of the FDA’s requirements for submission of clinical trial information.
- For research involving an IND (investigational new drug application) or IDE (Investigational device), the holder of the IND or IDE is the responsible party unless responsibility has been delegated to the principal investigator
NIH-funded studies
- The awardee or the investigator is responsible for registering the study and posting a copy of the informed consent form.
ICMJE requirements
- The ICMJE expects the authors to ensure that the study is registered appropriately.
If it is unclear who is responsible for registering an applicable clinical trial, investigators should consult with the sponsor, funding agency, and/or other study investigators to define who the responsible party will be.
Multiple institutions
The lead sponsor should be responsible. In the case then there is no sponsor, investigates must work together to determine who will be fulfilling the obligation.
What are the compliance timelines?
- Registration must be within 21 days after enrollment of first subject.
- For ICMJE, registration must be at or before the time of first subject enrollment.
- Study information must be updated no less than annually.
- If recruitment status, IRB status, or administrative information about the study (e.g. responsible party, study title, contact information) changes, registration must be updated within 30 days.
- When the study is closed to enrollment, no later than 60 days after the last study visit of any subject.
- Results submission and adverse events no later than 1 year after completion date of primary outcomes.